ABOUHAIDAR Rawan : Molecular Dynamics Simulation and Algorithmic Graph Theory of Freezing of Aqueous Alcohol Surfaces: Investigating the Isomer Effect
Résumé de thèse :
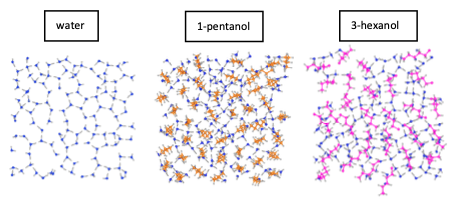
Surface Active Organic Compounds (SAOCs) are preferentially adsorbed at the surface and are more exposed to incoming radicals and gaseous compounds. Significant efforts have been conducted to understand the role of these molecules in atmospheric processes, such as the formation of cloud condensation nuclei. However, less attention has been given to the impact of SAOCs on freezing processes. The present study is motivated by nanodroplet freezing experiments [1,2] that invoked different partitioning of alcohols between the surface and the bulk. Here, classical molecular dynamics simulations have been performed to investigate the surface behavior of linear and branched alcohol isomers with different chain lengths, specifically 1-pentanol and 3- hexanol, and their impact on freezing. The results reveal a reduction in surface tension caused by the presence of the alcohol molecules at the interface. Moreover, as temperature decreases by approximately 50 K (283 K ® 192 K), surface tension increases and the solubility of both 3-hexanol and 1-pentanol increases. Furthermore, we used GaTewAY [3], a software that employs graph-theory-based methods to interpret and post-process molecular dynamics trajectories, specifically focusing on 2D-molecular graphs constructed from the H-bonds existing between molecules. Results demonstrate that both alcohols enhance the 2D-network of water molecules. Notably, branched alcohols promote pentagonal configurations within pure water, while linear alcohols exhibit a greater tendency towards hexagonal configurations involving five water molecules and one alcohol, thus enriching the cooling process. The present approach provides insights into how the molecular arrangement of different alcohol species within the interfacial zone affects freezing and ice formation.
[1] Sun, T.; Ben-Amotz, D.; Wyslouzil, B. E. Phys. Chem. Chem. Phys. 2021, 23 (16), 9991–10005.
[2] Sun, T.; Wyslouzil, B. E. J. Phys. Chem. B 2021, 125 (44), 12329–12343.
[3] Bougueroua, S.; Bricage, M.; Aboulfath, Y.; Barth, D.; Gaigeot, M.-P. Molecules 2023, 28 (7), 2892.
Doctorant : ABOUHAIDAR Rawan
Directeur de thèse : DUFLOT Denis, TOUBIN Céline
