Insights into the non-covalent interactions of hydrogen sulfide with fenchol and fenchone from a gas-phase rotational study
Monoterpenes constitute a significant family of molecules within the atmosphere, primarily emitted by plants and coniferous sources and make up 11% of the global biogenic organic compounds emitted into the Earth's atmosphere.1 The formation of Secondary Organic Aerosols (SOA) has been identified as influenced by humidity and water vapor, with higher concentrations positively correlating with increased SOA formation. Recent spectroscopic studies have been done to unravel the water-monoterpene interactions, shedding light on the initial stages of aerosol formation.2,3 Exploring the interactions of monoterpenoids with atmospheric species holds immense significance in the physical-chemistry of the atmosphere. Hydrogen sulfide (H2S), sourced from both natural and anthropogenic origins,4,5 emerges as a key player in shaping the Earth's atmosphere. The higher interest in this molecule compared to other sulfur compounds is due to its structure that resembles that of water.
With the aim of examining complexes involving H2S, two monoterpenes, Fenchol (EF) and Fenchone (FEN), were selected due to their analogous structures but differing functional groups. While their interactions with water have been extensively studied, the focal point shifts to discerning variations when compared to their water-containing counterparts. This effort seeks a deeper understanding of these molecular interactions and their implications.
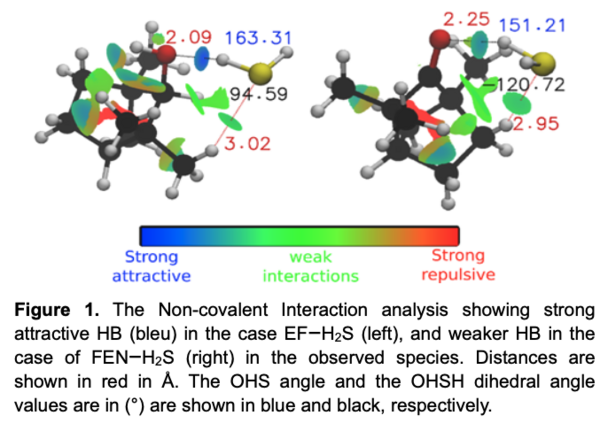
The identification and characterization of the interaction of H2S with Fenchol and Fenchone in the gas phase has been successfully accomplished (Fig. 1). Its comparison with water complexes reveals surprising aspects. The strong Hydrogen bond in EF–H2S forms a rigid structure similar to the case of EF–H2O. The much weaker HB in the case of FEN–H2S leaves the H2S unit dynamically quasi-free to rotate around its C2 axis as evidenced by the observed tunneling splitting of the lines. In addition, FEN–H2S is unique and very different from the two FEN–H2O conformers. Noticeably, only one FEN complex was observed with H2S while several mono-, di- and tri-hydrated complexes were evidenced.
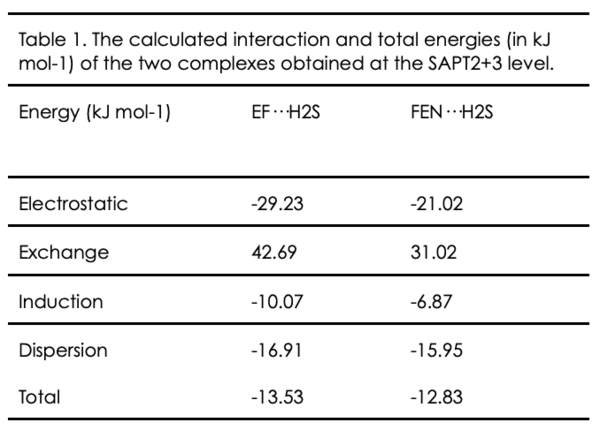
Our study shows that the hydrogen bond involving complexes with H2S is weaker to that with H2O. The obvious observation is that the principal HB is stronger in the case of EF–H2O (33.6 kJ.mol-1) than in the case of EF–H2S (15.4 kJ.mol-1). The same observation has been deduced for FEN–H2S, its interaction energy (∼6 kJ.mol-1) is weaker than that of both observed monohydrate complexes with water (>26 kJ.mol-1). The large amplitude motion of H2S in the case of FEN···H2S could be explained by the weakness of the hydrogen bond involved in its stabilization which is about 3 times lower than its value in the case of EF–H2S. To further confirm and understand the non-covalent interactions taking place, symmetry-adapted perturbation theory analyses were carried. It is one of the methods used for energy decomposition analysis, where it provides the decomposition of the total interaction energy into electrostatic, exchange, induction and dispersion energy terms. It can be seen that the electrostatic contribution to the total energy in the EF–H2S complex is higher than that in the FEN–H2S complex (Table 1). This can provide another proof that the hydrogen bonding in fenchol is stronger, and one more explanation for the fact that splitting is only observed in fenchone complex.
Références
1 Sindelarova, K. et al. Atmos. Chem. Phys. 14, 9317–9341 (2014), 2 Neeman, E. M. et al. Phys. Chem. Chem. Phys. 23, 2179–2185 (2021), 3 Chrayteh, M. et al. Phys. Chem. Chem. Phys. 23, 20686–20694 (2021), 4 Watts, S. F. Atmos. Environ. 34, 761–779 (2000)5 Ausma, T. et al. Frontiers in Plant Science 10, 743 (2019)
