Smart solution for CO2 capture using gas hydrates with promoters: an alternative for promoting carbon capture and storage
One of the MPI-team’s activities within the thematic ‘Environmental Sciences’ concerns the CO2 capture using ‘hydrate technology’. A unique set-up has been developed enabling in-situ assessment of clathrates technology’s performance parameters. Selectivity, CO2 recovery fraction, and recently normalized gas uptake can now be derived from the in-situ Raman spectroscopy analysis to probe hydrates properties. This breakthrough markedly differs from other set-ups currently used in hydrates research to characterize hydrates at macro-scale using absorption measurements and mass balance calculation. The originality lies here in the fact that our stand-alone optical spectroscopic technique provides high resolution signatures of guest molecules from which a self-consistent methodology was developed to reveal the relevant parameters of the hydrate system.
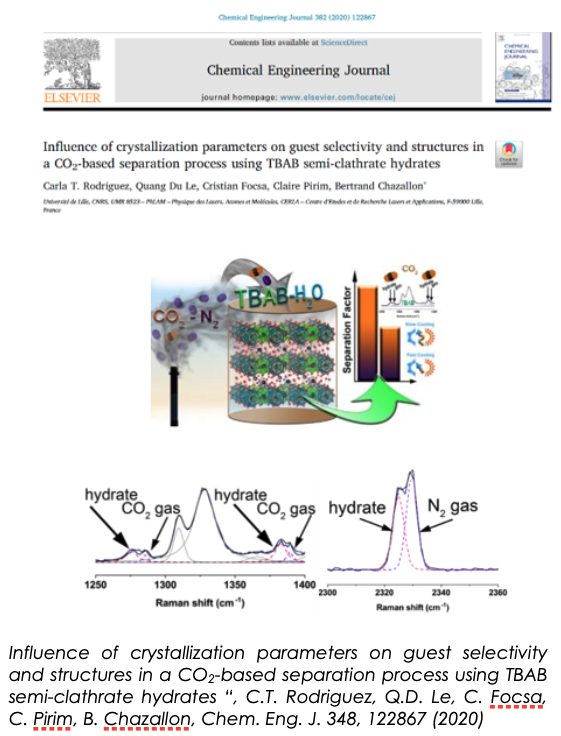
One of our main achievements was obtained on the separation of CO2-N2 gas mixtures using a quaternary ammonium salt (TBAB) as thermodynamic promoter, which allows a net reduction of the hydrate formation conditions from ~120 bar and 0°C in the pure water to ~35 bar and 15°C in the semi-clathrates. CO2 selectivity and capture were derived in these TBAB-based semi-clathrates. Best results in terms of selectivity were obtained using a slow-cooling protocol. Furthermore, the type A (tetragonal) structure observed in distinct protocols showed the best selectivity. These results can serve as a basis for developing the HBSP process for CO2 capture.
This work take place within the context of the INTERREG C2V projects involving the expertise of one academic partner (ULille) and five industrial partners (among them, ARCELORMITTAL (Gent), DOW Chemical BENELUX, and LANZATECH (USA)). The objective was to demonstrate the potential of reduction of GHG emissions in the steel sector by 30%, by implementing a cost efficient breakthrough solution for the separation of CO2 & CO unavoidably emitted. This was achieved by processing in a pilot line carbon rich gas into 2 streams, one rich in CO and another one in CO2 that could be valorised into promising chemical building blocks. The project intended to consider the reuse of any by-products to further induce fossil fuels’ replacement and GHG emissions reductions.
