2) Environmental Sciences
The physical properties, chemical reactions, evolution, and characterisation of either particles or molecules involved in atmospheric processes are tackled from a physicochemical perspective using non commercial home-built instrumentation. The research activities of interest to the understanding of processes occurring in the gas–phase address the identification of micro-solvation sites with water, the structural changes induced by micro-hydration, the complexation and reactivity of troposphericrelevant gaseous species with sulfur compounds, the development of new instruments, and the refining of posttreatment (line profiles) or simulation of high-resolution molecular spectra. Examples of the group's research activities related to this theme are described below.
a. Gas-phase physico-chemical properties of volatile organic compounds.
The structural properties of volatile organic compounds (VOCs) are investigated. These studies are supported by the LabEx CaPPA and the CPER CLIMIBIO and ECRIN. Three objectives are considered:
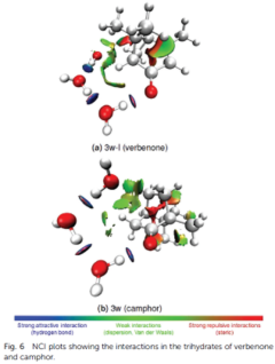
- Identify the position of micro-solvation sites with water. We aim to show how water could interact with the oxidation products of volatile organic compounds, minority compounds of the atmosphere, in the gas phase, especially with monoterpenoids (alhedydes and bicyclic ketones comprising 10 carbon atoms). For example, mono-, di- and tri-hydrates were characterized for verbenone and camphor, using quantum chemical calculations and Fourier-transform microwave spectroscopy in a pulsed supersonic jet.
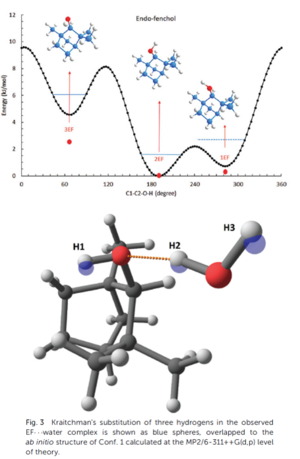
- Study the structural changes of the parent species associated to micro-hydration. For example, we have shown how a hydration process changes (case of fenchol), or does not change (case of myrtenol), the conformational landscape of the host molecule, at the level of a functional group, in terms of relative energy. We have emphasized the importance of hydrogen bonding in stabilizing the lowest energy species.
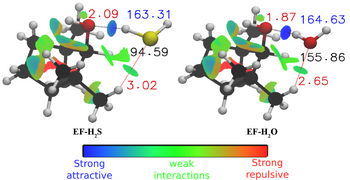
- The study of solvent species is extended from water to hydrogen sulphide (H2S), the latter being ubiquitous in the atmosphere. They are emitted by human activities (combustion processes), but natural sources provide the largest amounts of sulphur compounds. They play a crucial role in the formation of secondary organic aerosols (SOAs), by transforming organic volatile compounds (VOCs) mainly emitted by terrestrial vegetation into the condensed phase, in particular through weak interactions (hydrogen bond, London dispersion forces). Note that hydrogen bond involving an atom of sulphur is expected to be weaker than that involving an oxygen atom. We performed the first quantitative characterisation of the hydrogen bond of H2S with biogenic volatile organic compounds. Our work on fenchol and fenchone indicated that sulphur behaves differently from oxygen during the micro-solvation process.
b. Line profile of atmospherically relevant molecules.
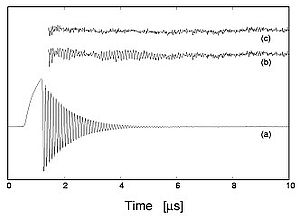
The influence of molecular velocity on collision-induced transition frequency shifts is studied by analysing experiments using coherent transient regime techniques (optical precession) on the rotational transition at 86 GHz of HC15N. This effect, characterized by a temporal variation of the optical precession frequency, is responsible for an asymmetry of the absorption lines. It is perfectly explained within the framework of the hypergeometric model of velocity dependence of relaxation rates, which is impossible with the quadratic model used in the databases. In collaboration with the LPCA of ULCO (Dunkirk), and the laboratory of Spectroscopy and Molecular Dynamics of the Ecole Nationale Supérieure d'Ingénieurs de Tunis (Tunisia), the study of collisional broadenings of submillimeter transitions of H2S induced by H2S and N2 made it possible to highlight the velocity dependence of molecular collision rates. In collaboration with the UTINAM Institute in Besançon, and the V.E. Zuev Institute of Atmospheric Optics in Tomsk (Russia), a similar study has been performed on the collisional broadening of submillimeter transitions of CH3CN induced, among others, by N2, O2 or rare gases.
c. Reactive halogenated species
Halogenated compounds have a major impact on the chemical composition of the atmosphere, but most studies on halogenated species do not include iodine. Several key iodine-containing species, identified as being released into the atmosphere with a high probability, either naturally or anthropogenically, have been studied for the first time: the photolabile dihalomethane CH2BrI from the INOx family (x = 1-3). This family can be considered an important temporary reservoir for iodine and nitrogen oxides. These results, supported by LabEx CaPPA, were achieved through significant theoretical and experimental collaborations with D. Duflot (PCMT team) and H. Ozeki (Toho Univ., Japan).