SHAABAN Tamara: Theoretical Modeling of Protactinium Physico-Chemical and Spectroscopic Properties
Résumé de thèse :
The physical and chemical properties of solvated actinide complexes, including their speciation, bond nature with the surrounding environment, and thermodynamics and spectroscopic properties, hold significant implications for societal and industrial applications. The coordination of the actinides with ligands [1] affects these properties. Researchers, both experimental and theoretical, are interested in the coordination and properties exhibited by the f-elements with ligands.
Among the actinides, protactinium (Z=91) keeps on being specific because, depending on its oxidation state it can behave as ‘’f element” (Pa(VI)) or “d element” (Pa(V)). In solution, Pa(V) is dominating because Pa(IV) is unstable and can be directly oxidized to Pa(V) unless a strong reducing agent is present [2]. Pa(V) can exist in solution as Pa5+ and in some specific solutions it will form PaO3+ [3], but apparently it does not form the actinyl moiety PaO2+ which is not the case of their heavier neighbour’s uranium, neptunium and plutonium.
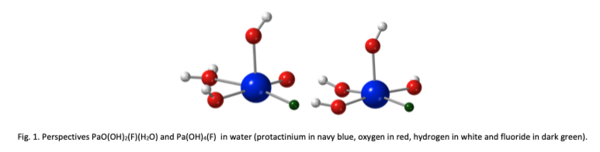
In this work, with the use of the state-of-the-art quantum calculations, we investigate the two possible forms of Pa(V), namely PaO3+ and Pa5+ and the influence of coordinated ligands in order to determine the suitable experimental conditions to tune their relative stability. For that we consider two different stoichiometrically equivalent complexes PaO(OH)2(X)(H2O) and Pa(OH)4(X) where X=OH- , F- (see Fig. 1), Cl-, Br-, I-, NO3-, NCS-, C2O42- and SO42- and compare their relative stabilities.[4]
It was supported by grants funded the French National Agency for Research (ANR-21-CE29-0027, LABEX CaPPA/ANR-11-LABX-0005-01 and I-SITE ULNE/ANR-16-IDEX-0004 ULNE).
References
[1] K.Binnemansetal.,Chem.Rev.,107,2592(2007).(DOI:10.1021/cr050979c)
[2] N.Baniketal.,DaltonTrans.,45,453(2016).(DOI:10.1039/C5DT03560K)
[3] C.LeNaouretal.,Inorg.Chem.,44,9542(2005).(DOI:10.1021/ic0512330)
[4] T.Shaabanetal,Chemistry-AEur.Journal,30(2024).(DOI:10.1002/chem.202304068)
Doctorant : SHAABAN Tamara
Directeur de thèse : VALLET Valérie, RÉAL Florent
